f orbital electron configuration|electron configuration orbital diagram : Cebu The elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of . Tingnan ang higit pa What Time Is It In South Carolina, United States? 3:49:44 PM. Friday, August 30, 2024. Eastern Daylight Time (EDT) -0400 UTC. UTC/GMT is 19:49 on Friday, August 30, 2024. Difference from your location: 1 hour ahead of Unknown, Kansas. DST. Time Zone Map. Daylight Saving Time. Starts On March 10, 2024 at 02:00 AM. Set Your Clock. Ahead 1 .
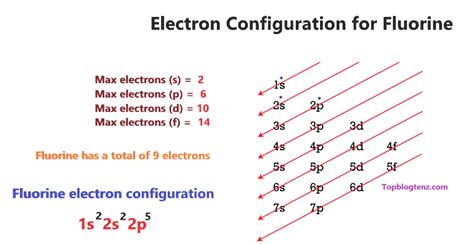
f orbital electron configuration,Atoms can jump from one orbital to another orbital in the excited state. This is called a quantum jump. The ground-state electron configuration of fluorine is 1s2 2s2 2p5. We already know that the p-subshell has three orbitals. The orbitals are px, py, and pzand each orbital can have a maximum of two . Tingnan ang higit paf orbital electron configurationThe total number of electrons in fluorineis nine. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in fluorine in . Tingnan ang higit pa

After arranging the electrons, it is seen that the last shell of the fluorine atom has seven electrons. In this case, the valence electrons of fluorineare seven. The elements that have 5, 6, or 7 electrons in the last . Tingnan ang higit paThe elements in group-17 of the periodic table are called halogen elements. One of the elements in group-17 of the periodic table is fluorine and the first element in group-17 . Tingnan ang higit paThe elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of . Tingnan ang higit pa
The orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. The orbital letters are associated with the angular momentumIn writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s .
Wayne Breslyn. 764K subscribers. 77K views 4 years ago Electron Configurations. In this video we will write the electron configuration for F-, the Fluoride ion. We’ll also look at why.
Seven f orbitals: 2 s orbital electrons: 6 p orbital electrons: 10 d orbital electrons: 14 f orbital electronsThe s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f subshell has 7 .

The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict and .
The electronic configuration for the Fluorine ion is 1s22s22p5 and in this configuration, Fluorine needs 1 electron so as to complete the 2p orbital. This 1 electron by the Fluorine will be acquired .
electron configuration orbital diagraml,the azimuthal quantum number or angular momentum quantum number,tells you the shape of the orbital the electron is in. l can be any value from 0 to n-1. l=0 is s-orbital, which is .
f orbital electron configuration|electron configuration orbital diagram
PH0 · how to find electron configuration
PH1 · ground state electron configuration fluorine
PH2 · full orbital diagram for f
PH3 · f ground state electron configuration
PH4 · electron orbitals chart
PH5 · electron configuration orbital notation worksheet
PH6 · electron configuration orbital diagram
PH7 · electron configuration calculator
PH8 · Iba pa